Results from the WHITE4 study of X-Bolt Orthopaedics’ hip fracture treatment, comparing the X-Bolt® XHS vs. the gold standard Sliding Hip Screw (SHS), indicate that patient wellbeing outcome scores at four months were better in more than 90% of XHS vs. SHS patients, rising to 95% favouring XHS when crossovers (XHS-allocated who actually received SHS) are excluded and reference is made to baseline pre-injury scores.
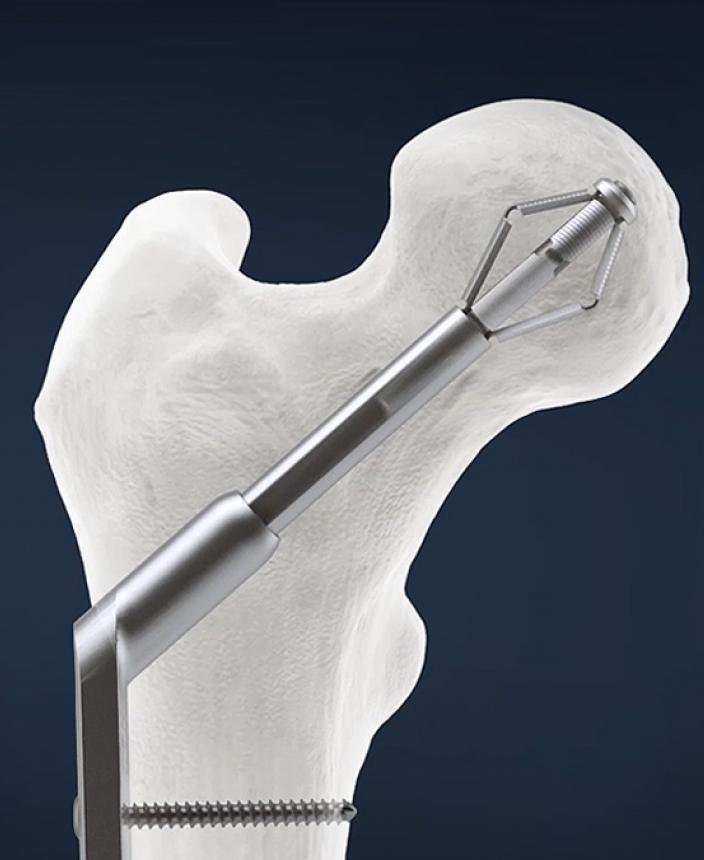
SHS fixation is an established treatment of trochanteric hip fractures. XHS differs only by the nature of fixation in the femoral head, using an expanding bolt instead of a lag screw. WHITE4 was the world’s largest randomized trial in hip fracture fixation across 10 U.K. specialist orthopedic centers.
Reoperation rates, including femoral head cut-out, were the lowest ever recorded for a trial of this size (1,140 subjects). Cut-out is a failure of implant anchorage that causes the lag screw to literally cut out of the bone and into the hip joint.
Having achieved FDA clearance of its hip plating and nailing systems in 2018, the company recently expanded its product portfolio. It has incorporated its technology into other long bone nailing systems, as well as an expandable spinal pedicle device. The company has also recently improved the XHS expanding bolt, making it even easier to use, with a shorter tip-apex distance and compatibility with generic SHS barrel plates. X-Bolt Orthopaedics’ expanded portfolio includes:
- Pendulum™ humeral nail with a distal expansion zone and strong humeral head fixation with an expanding bolt and angled screws
- Springboard™ tibial nail featuring a strut to allow more proximal fixation and a micromotion suspension mechanism
- Karibu™ femoral nail with double-ended X-Bolt for distal femur fixation, allowing greater weight-bearing
- The curved Metro™ jig with Flexi-chain drive, allowing surgeons to operate ‘around a corner’
The company is currently raising finance to accelerate commercialization of the entire portfolio in the U.S. and globally.
X-Bolt Orthopaedics’ CEO, Dr. Brian Thornes, said, “This was a large trial in a very frail patient group, whose main desire is to stay out of the hospital and to maintain their independence. The data confirms the outstanding safety profile of the X-Bolt, which virtually eliminates ‘cut-out’ as an issue. I am also delighted the clinical impact translates into tangibly better outcomes for a significant number of patients.”




