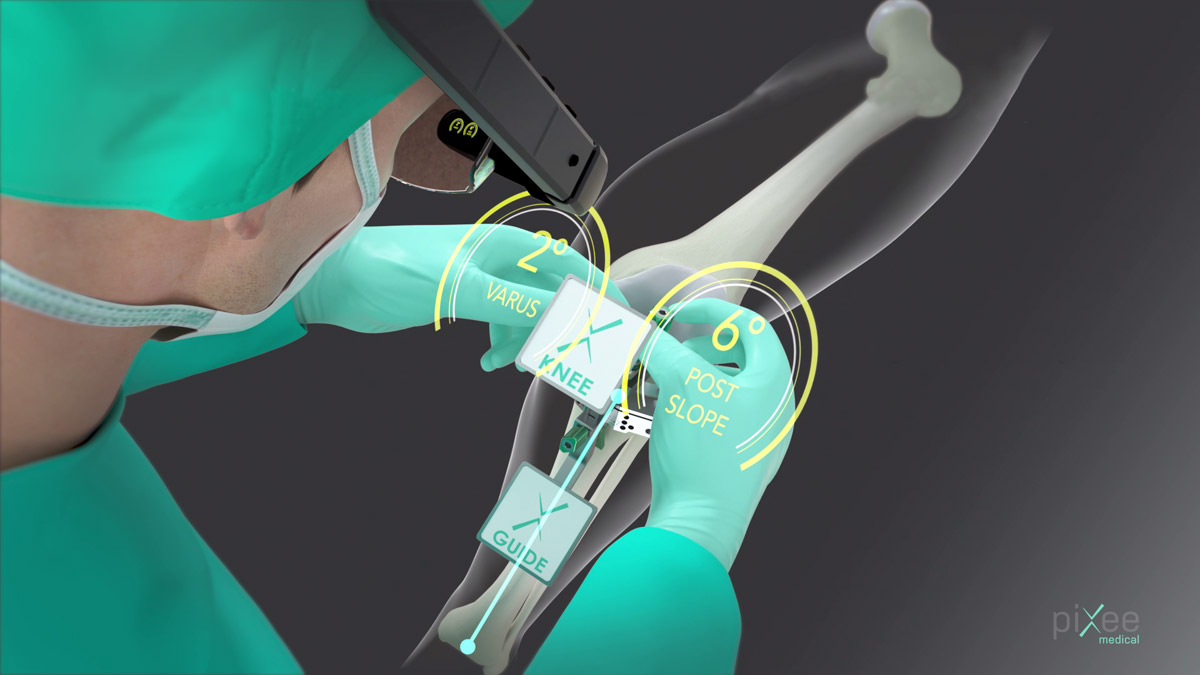
Pixee Medical received FDA 510(k) clearance to market its Knee+ AR computer assisted orthopedic solution.

Knee+ is designed to help orthopedic surgeons perform surgeries better and faster by providing real-time positioning of instruments, right in their field of view. Knee+ is intuitive and requires minimal training, since it does not change the overall technique for 90% of surgeons who use a conventional technique but have never utilized navigation or robots.
Knee+ comprises proprietary software using computer vision and artificial intelligence algorithms that runs on connected smartglasses, with no bulky capital equipment or disposables required.
The first Knee+ procedure was performed in 2020. Pixee Medical began commercialization in Europe and Australia in 2021, with more than 60 systems sold in 1Q21.
“FDA’s clearance of Knee+ is an important step forward, as the USA represents 50% of the worldwide market. We plan to quickly expand our platform to perform hip and shoulder replacements” states Sébastien Henry, Founder and CEO of Pixee Medical. “In addition, our platform is designed to become the cornerstone of data acquisition and exchange during surgery as well as a plug-and-play hub for accessories like connected instruments, robotic arms and wireless tools.”