Graphene Flagship spin-off, INBRAIN Neuroelectronics, is developing graphene-based brain implants to treat brain disorders.
In Europe, there are 165 million living with a brain disorder, and one in three people are predicted to suffer with some neurological disorder during their lifetime. Each year, the cost of treating brain disorders equates to 35% of Europe’s total disease burden — that’s almost €800 billion.

INBRAIN is a spin off from the Graphene Flagship, one of the largest European research initiatives, and its partners the Catalan Institute of Nanoscience and Nanotechnology (ICN2) and the Catalan Institution for Research and Advanced Studies (ICREA), in Spain. Established in 2019, INBRAIN uses technology developed by Graphene Flagship partners ICN2, ICREA and the University of Manchester to develop smart devices for patients with neuronal disorders, like Parkinson’s or epilepsy.
INBRAIN’s technology is built around graphene electrodes which decode with high fidelity neural signals from the brain and then can produce smart therapeutic responses specific to the clinical condition of each patient.
Clinicians have long used electrode arrays to monitor and to stimulate brain activity. But, the performance of commercially available technologies, based on metals like platinum, drops significantly when miniaturised. Existing clinically-used electrodes are therefore very large and unable to accurately stimulate the affected area or record brain activity in detail. As a result, despite the enormous potential of neuromodulation to treat a range of brain disorders, current efforts have proved largely ineffective, with many causing significant side effects.
Graphene’s properties enable the electrodes to be miniaturised while still performing excellently. Graphene-based implants can support many more electrodes than traditional electrode arrays, and therefore are capable of high-resolution recording and stimulation. This results in mapping of brain activity with unprecedented spatial and temporal resolution and, at the same time, brain stimulation with unique precision.
A smartest and low-invasive neural interface, INBRAIN’s device can be used over large areas of the cortex without interfering with normal brain function or can be integrated into probes reaching deep structures of the brain. Powered by artificial intelligence and using big data, the implants can read and modulate brain activity, detect specific biomarkers and trigger adaptive responses to deliver optimal results in personalised neurological therapies.
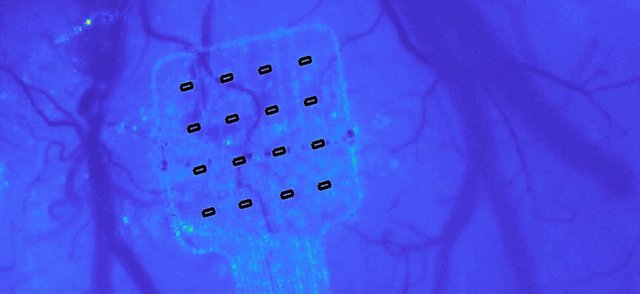
“The high-density bi-directional graphene dots in our brain interfaces collect real-time high-resolution brain signals and identify relevant biomarkers,” explained Carolina Aguilar, CEO of INBRAIN Neuroelectronics. “We link these to symptoms to generate a therapeutic solution. This entire process can be done through machine learning algorithms that eventually make the process automatic, delivering a true neuroelectric therapy free of the side effects associated to the current therapies.
“As we collect more securitised and anonymised data, we can create better and smarter algorithms and therefore more effective therapeutic solutions.”
INBRAIN’s technology has been validated in in-vitro and in-vivo biocompatibility, as well as toxicity tests. It has been used successfully in studies on small animals and will be further validated through tests on large animals to ensure they are safe and superior to current solutions using metals.
To achieve this ambition a team that has been put together, combining technical expertise and business acumen. It includes Graphene Flagship figures Jose Garrido, Kostas Kostarelos and Anton Guimera, neurotechnology experts from Philips, Medtronic and other start-ups such as Bert Bakker and Michel Decre, and neurotech business knowledge thanks to former Medtronic global director Carolina Aguilar. The team continues to grow with key neuro-engineering talent in Europe.
INBRAIN is currently working to ensure patient safety and to comply with necessary pre-clinical work and clinical regulatory milestones. The company plans to start its first in-human studies this year.






