The FDA cleared its first surgical robot designed for transvaginal hysterectomies, a minimally invasive option that aims for a faster recovery time compared to procedures that require a larger abdominal incision.
Memic Innovative Surgery’s Hominis system is intended to assist with the removal of the uterus, along with one or both of the fallopian tubes and ovaries, in cases where there is no cancer present, as well as with the removal of ovarian cysts.

The device includes a pair of articulated surgical instruments inserted through the vagina, designed to replicate the motions of a human arm with shoulder, elbow and wrist joints. An additional arm guides a laparoscopic video camera through a small, separate incision, to help visualize the internal procedure.
As part of its de novo clearance, the FDA is requiring Memic to provide comprehensive training programs for surgeons and operating room staff before they can operate the device.
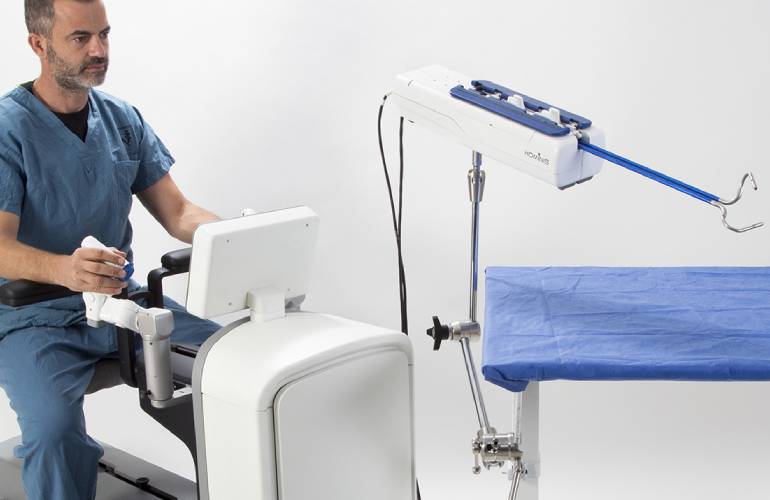
“We are pleased to receive FDA de novo authorization of our Hominis system, which offers a small, cost-effective and less invasive option over current robotic instruments limited to straight shaft and single wrist designs and controlled with large, complex and expensive equipment,” Memic CEO Dvir Cohen said.
The company estimates that about 600,000 hysterectomies are performed each year in the U.S., though the transvaginal approach is only used about 16% of the time.
“This authorization is also just the beginning; it opens the door for our novel system to expand to additional indications that, until now, have been off-limits to robot-assisted surgery,” Cohen added. The company is also investigating the use of its system for gastrointestinal, thoracic, urologic and general surgery procedures.
A clinical study of the Hominis system included 30 participants of various ages, body types and comorbidities, with all hysterectomy procedures being successfully completed without complications, and without having to revert to a different surgical approach, the FDA said.




